Rapid detection of Carbapenemase-Producing Enterobacteriaceae (CPE)
December 8, 2020
A novel method to detect carbapenemase activity in bacterial cell suspension
Key Word : Infectious disease, Discovery tool, Medical device & Software, antibiotic target, carbapenem
Background / Context / Abstract:
Carbapenem-resistant Enterobacteriaceae (CRE) can be classified in two groups; one is CP-CRE (CPE) which has plasmids encoding the carbapenemase gene, and the other is non-CP-CRE which acquires resistance to carbapenem by genomic mutation.
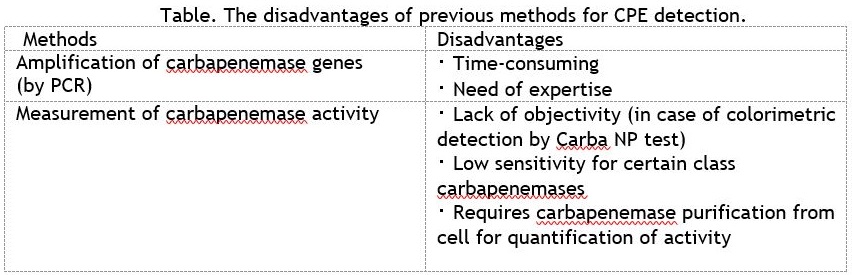
Rapid detection of CPEs is important for the appropriate therapeutic approach and infection control measures in clinical settings, however previous methods are not suitable for the reason described below.
Technology Overview:
Researchers at Osaka University have established a novel method to detect carbapenemase activity in bacterial cell suspension. This technology can rapidly detect CPEs by a simple and concise method, and can also detect OXA-type CPEs which are widely disseminating in Europe and the Middle East.
Benefits:
・Rapidity: Detection of CPE within 30 to 60mins.
・Objectivity: Carbapenemase activity is readily quantifiable
・High sensitivity and specificity: OXA-type carbapenemase is also detectable
・Low cost: Easily and simply manufacturing for a disposable kit or device
Further Details:
Carbapenemase activities of 55 different clones (non-CPE:13, CPE:42) were measured.
CPEs were detected in 30 to 60 min. with sensitivity and specificity of 100%.
Potential Applications / Potential Markets:
・Diagnosis
・Medical devices
State of Development / Opportunity / Seeking:
・Available for exclusive and non-exclusive licensing
・Exclusive/non-exclusive evaluation for defined period (set up for options).
・Collaborative/supportive research
IP Status:
WO2018/181997( PCT applied in Japanese )
Contact:
![]()