Photocatalytic resins that produce hydrogen peroxide
September 13, 2021
Background / Context / Abstract:
Hydrogen peroxide (H2O2) is a versatile oxidant for pulp bleaching and disinfection, and is manufactured by an energy-consuming anthraquinone method using hydrogen and oxygen gasses by multistep reactions.
Energy-saving H2O2 synthesis driven by sustainable energy (solar energy) and earth abundant resources such as water and air is therefore desirable.
Technology Overview:
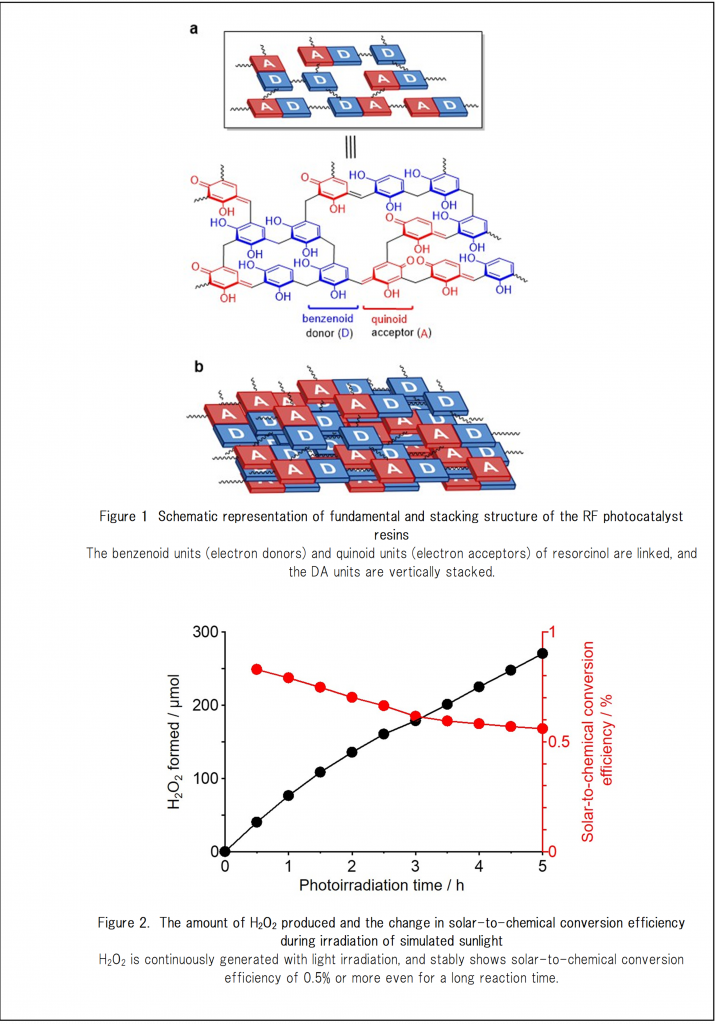
The H2O2 production can be realized by using inexpensively-manufactured organic semiconductor resins that is cross-linked with resorcinol derivatives as photocatalysts. Resorcinol-formaldehyde (RF) resins, when prepared by high-temperature hydrothermal synthesis, are suspended in water and exposed to visible light, oxidation of water and two-electron reduction of oxygen in water can be efficiently advanced to produce H2O2 at a very high light-energy conversion efficiency.
Benefits:
Advantages:
– Low cost: this technique requires the preparation of a metal-free organic semiconductor resins from inexpensive raw materials and exposure of the catalyst to visible light (sunlight).
– Hydrogen peroxide (H2O2) can be stably supplied at a high light-energy conversion efficiency.
Data:
– The invented RF resins have characteristics of n-type semiconductors and exhibits a band structure suitable for oxidation of water and two-electron reduction of oxygen. Hydrogen peroxide can be produced by photoexcitation with a long wavelength of 600 nm or more.
– The RF catalyst (10 mg) was prepared by hydrothermal synthesis at 250°C and adhered to a transparent substrate. When the catalyst was exposed continuously to fluorescent light (60 W) in water, hydrogen peroxide was efficiently produced, saturating at about 100 ppm. (Note) Few microorganisms such as E. coli can grow in a 100-ppm hydrogen peroxide solution.
– Although the illumination was continued for not less than 500 hours, the catalyst was not degraded and the catalytic activity (production of hydrogen peroxide) was not changed at all.
Publications:
– Shiraishi, Y et al., “Resorcinol–formaldehyde resins as metal-free semiconductor photocatalysts for solar-to-hydrogen peroxide energy conversion.” Nature Materials (2019) 18, 985–993.
https://doi.org/10.1038/s41563-019-0398-0
Potential Applications / Potential Markets:
Paint with photocatalytic activity;
Container for cell culture/storage;
Antibacterial resin products (childcare/nursing care products, sanitary products such as containers for storage of nail/contact lens, household products for kitchen, toilet, bathroom, etc.) and Others
State of Development / Opportunity / Seeking:
・Available for exclusive and non-exclusive licensing
・Exclusive/non-exclusive evaluation for defined period (set up for options)
・Collaborative/supportive research
※Seeking
1. Development partner
2. Licensing
IP Status:
WO2018/074456(Issued: JP,US)
Figures:
Contact:
![]()