Extremely stable supramolecular sulfur polymer
May 19, 2022
Background / Context / Abstract:
There are calls to make effective use of the 7 million tons of sulfur put into landfill each year. Sulfur is decomposed and unstable at room temperature because the eight-membered ring is broken, generating radicals. Further, sulfur polymers also demonstrate poor workability because they are insoluble in general-purpose, organic solvents.
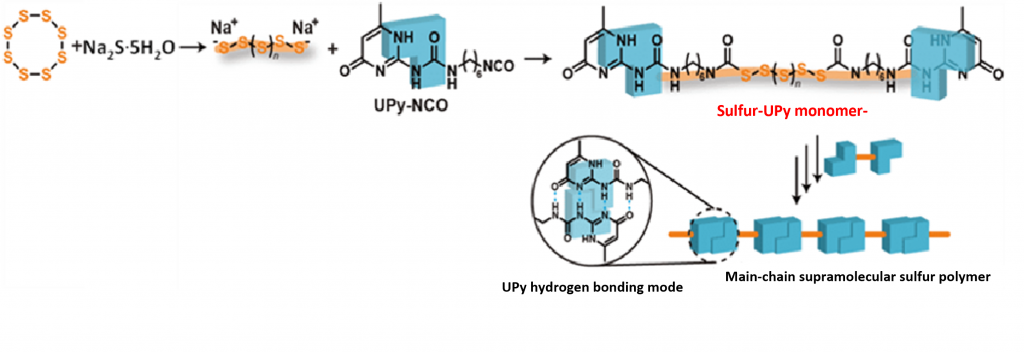
By adopting a supramolecular chemical approach, the inventor has achieved the stabilization of sulfur polymers. More specifically, a monomer in which noncovalent binding sites (hydrogen bond) are introduced to both ends of a straight chain sulfur is produced, and a supramolecular sulfur polymer with a large molecular weight is created by connecting the noncovalent binding sites. Radical backbiting at the end of the sulfur polymer (one of the causes of sulfur polymer degradation) is suppressed by the noncovalent binding sites at both ends. Sulfur molecules exposed in the straight chain may exhibit specific electrical or optical properties. The reversible cross-linking structure of the noncovalent sites may result in environmentally friendly materials that can be decomposed after use and reused by recombination formation.
Technology Overview:
Decomposition of the polymer is difficult due to its supramolecular structure while maintaining its solubility in organic solvents.
Benefits:
Advantages
- We anticipate that the sulfur molecule exposed in the straight chain demonstrates electro or optically unique physical properties.
- It is possible that it can be used as a material for lithium-sulfur batteries
- It may be possible to manufacture high refractive index optical plastics with a higher sulfur content.
Potential Applications / Potential Markets:
- A positive electrode material for lithium-sulfur batteries with high stability and good charge-discharge cycle characteristics.
- A plastic material with low environmental impact that can be manufactured from sulfur waste and decomposed after use.
State of Development / Opportunity / Seeking:
・Available for exclusive and non-exclusive licensing
・Exclusive/non-exclusive evaluation for defined period (set up for options)
・Collaborative/supportive research
※Seeking
1. Development partner
2. Licensing
IP Status:
PCT applied in Japanese
Figures:
Data
Sulfur was ring-opened by mixing Na2S・5H2O and elemental sulfur in water at room temperature for 24 h. A chloroform solution in which UPy-NCO was dissolved was added and stirred at room temperature for 1 day. The precipitate formed between the interfaces was recovered and column chromatography used to obtain the sulfur-UPy monomer. We then synthesized the supramolecular sulfur polymer.
Contact:
![]()